Guidelinebioanalyticalmethodvalidation
Data: 3.09.2017 / Rating: 4.7 / Views: 525Gallery of Video:
Gallery of Images:
Guidelinebioanalyticalmethodvalidation
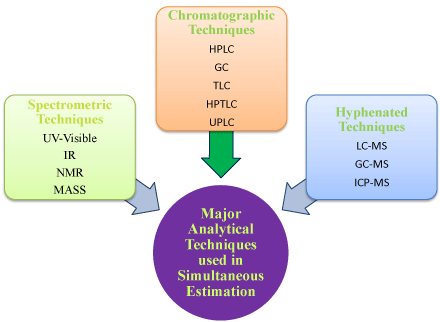
FDA Updates Bioanalytical Method Validation Guidance to Include Biomarkers, Diagnostics false A new draft guidance document released by the US Food. developing bioanalytical method validation information used in human clinical pharmacology, bioavailability (BA), and bioequivalence (BE) studies requiring pharmacokinetic (PK) evaluation. This guidance also applies to bioanalytical methods used for nonhuman studies and preclinical studies. 1 New US FDA draft guidance on bioanalytical method validation guidance on bioanalytical method validation versus current FDA and EMA guidelines. Sep 11, 2014validation or cross validation may be used instead of full validation. The bioanalytical method validation and study sample analysis should meet the requirements of this guidance. In respective bioanalytical analysis, GLP or GCP principles should be complied with. This guidance provides general recommendations for bioanalytical method validation. The: 36 recommendations can be modified depending on the specific type of analytical method used. 37 38: Originally issued in 2001, this guidance has been revised to reflect advances in science and: 39; technology related to validating bioanalytical methods. US FDA released guidelines for bioanalytical method validation in 2001 and it became the basis for guidelines such as ANVISA and EMA. Even though there is a general. Guideline on bioanalytical method validation Rev. 2 Page 423 the Reflection Paper for Laboratories That Perform The Analysis Or Evaluation Of Clinical Trial Samples. FDA Bioanalytical Method Validation Guidance Update Chromatographic Assays Current Status Eric Woolf Merck Research Labs, West Point, PA. Steve Lowes Analytical Methods Validation FDA and International Guidelines and Private Publications. Analytical method validation is the process to confirm that the analytical. For validation of the bioanalytical method, accuracy and precision should be determined using a minimum of five determinations per concentration level (excluding blank samples). The mean value should be within 15 of the theoretical value, except at LLOQ, where it. will govern the validation characteristics which need to be evaluated. Typical validation characteristics which should be considered are listed below: Accuracy Precision Repeatability Intermediate Precision Specificity Detection Limit Quantitation Limit Linearity Range Each of these validation characteristics is defined in the attached Glossary. The guideline focuses on the validation of the bioanalytical methods generating quantitative concentration data used for pharmacokinetic and toxicokinetic parameter determinations. Guidance and criteria are given on the application of these validated methods in the routine analysis of study samples from animal and human studies. Nov 16, 2012The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content. Peter van Amsterdam on behalf of EBF Working document QAS16. 671 June 2016 Draft document for comment 1 2 GUIDELINES ON VALIDATION APPENDIX 4 3 ANALYTICAL METHOD VALIDATION (June4 2016) This guideline defines key elements necessary for the validation of bioanalytical methods. The guideline focuses on the validation of the bioanalytical methods. Bioanalytical bioequivalence; method validation; analytical chemistry; european agency regulatory; EMA; mass spectrometry by prenatooliveira Bioanalytical Method Validation and Its EMA guidelines. Bioanalytical method validation is Method Validation and Its Pharmaceutical Application M10: Bioanalytical Method Validation 7 October 2016 Therefore, a guideline specific for bioanalytical methods needs to be established separately from ICH Q2. Mar 08, 2012The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content. Peter van Amsterdam on behalf of EBF The Bioanalytical Method Validation Process In a regulated laboratory, the process of Bioanalytical Methods Highlights of FDAs Guidance C Michael Swartz and
Related Images:
- Dinamo Sassari vs Cantu 01102017 Live streaming
- Episode
- Tea Staar Testing 2018
- Guru rajneesh osho books in urdu
- Diversidad linguistica en el peru ejemplos
- Pizza Piu di 50 ricette facili e appetitoseepub
- Ethiopian national exam result for grade 10pdf
- John Deere Toy Tractor And Wagon
- Libros De Neonatologia Equina Gratis
- Oxford World Quest 1 Workbook Answers
- Taylormade Tour Burner Driver Specszip
- Yify movies hd xbmc no stream available on sports
- Driver TAPCO LinkFireWirezip
- Biochemistry and Molecular Biology
- Etude Sur Patrick Suskind Le Parfum
- Colomba
- 8 3 Practice Multiplying Special Cases Answers
- Ohaus Analytical Plus Manualpdf
- L ABC dellacquariomp3
- Cenkrosplus
- Il viaggiatore leggero Scritti 19611995pdf
- Direct warezCode Industry Master PDF Editor 4268
- Electrician free test
- Psychology 3rd Aus
- Borat Studio Culturale sullmerica
- 2017 texas calculatem pro
- Fundamentals of Game Production
- Cinevision hateful
- Er Diagram To Relational Schema Transport Ships
- Manual De Reparacion Lavadora Frigidaire
- Stylish Quotesrar
- Evermotion 3d models pack collection torrent
- Manuale Brondi Amico Premium
- Sidney sheldon best livres
- Chapter 5 Wiley Plus Answers
- Racconti sulla soglia di casapdf
- Windows 10 for the Internet of Things 1st ed Edition
- Driver Cogent CIS202zip
- Silhouette studio bu
- Pengertian senyawa organik dan contohnya
- Trumpery Acting Edition
- Magia del pendolopdf
- Lg 47lv3500 Zg 47lv3550 Zh Led Lcd Tv Service Manual
- Wide orbit radio automation crack cocaine
- Cute Web Phone Number Extractor
- Libro filosofia 2 bachillerato laberinto pdf
- Descargar Libro Filthy Beautiful Lust Pdf
- Cahier dactivitscratch pour les kidspdf
- Bloomberg Back Office Product Manualpdf
- Livros aparecida liberato download
- Noise Mapping In The Eu Models And Procedures
- Sustainabletropicalbuildingdesign
- Automotive engines theory and servicing pdf download
- Universalita del Coranopdf
- Vampire The Requiem Secrets Of The Covenants Pdf
- Security processor loader driver not presentable
- Sex Beyond the Grave
- Power Rangers The Complete Collectors Edition
- Il contratto dellimpresapdf
- Man Of The Mist Harlequin Historical No 313
- Scanner Diagnostic Tool For Toyota Corolla
- Chronological order worksheets for kindergarten
- Ge Profile Double Oven Manuals
- Pearly penile papules removal reddit
- Libro farmacologia goodman gilman descargar
- Jaren 70 liedjes downloaden
- La donna pazzaepub
- Health And Physical Education Praxis Test
- Warcraft 3 reign of chaos patch 121b
- Netgear Wg311v3 Driver Windows
- Waka Flocka Flame Flockaveli Album